What is water hardness, what types of hardness there are and what units are used to measure it, we analyzed in the previous article “Water Hardness”. Now we have to figure out how to find out the hardness of water at home. And choose from the existing methods of the most suitable for us.
How to find out the hardness of water using Trilon “B”

It is by far the simplest and safest of the accurate quantitative analytical chemistry methods for determining water hardness. But easiest for an analytical chemist doesn’t mean easiest for an aquarist, even if they are a pro. Not to mention beginners or people who just want to have at home a beautiful aquarium, arranged with their own hands, without turning the apartment into a chemical laboratory. The method is certainly good and deserves attention. On sale there are sets of chemical reagents for determining the hardness of water. This method is accurate and, therefore, can serve to check and calibrate less complex methods, which, due to their simplicity, can be more often and more easily used in the home. However, the complexity of the method and the need for additional chemical equipment reduces its use in the home.
TDS-meter and how to find out water hardness with it
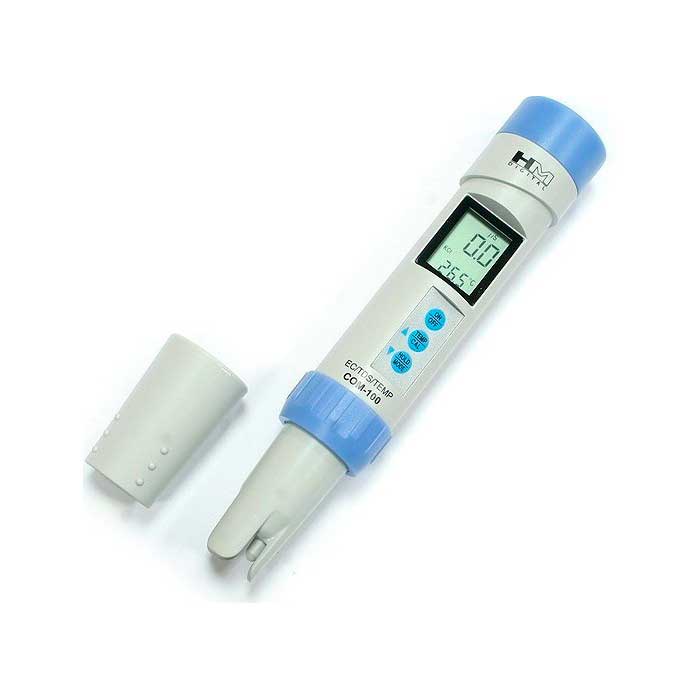
A conductometer (TDS-meter) is an electronic instrument that measures the conductivity of water, which is higher the more salts are dissolved in it. This is why it is sometimes also called a salt meter. It is by and large impossible to measure the total hardness with this device. But by its readings can indirectly judge the value of the total hardness, using special tables or formulas for matching the readings of the device to the total hardness, which are given in its instructions for use. In particular, in the book by I.G. Homchenko “Modern Aquarium and Chemistry” provides a table on which you can judge the total hardness of water by its electrical conductivity.
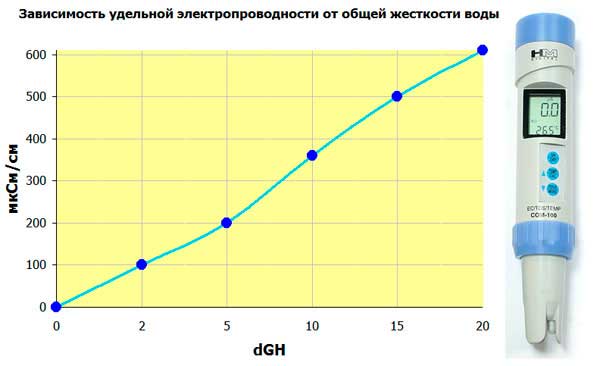
The method impresses with its simplicity. But when using it, it is necessary to take into account that it is an indirect measurement of total hardness. The readings of the device strongly depend on the temperature of the solution (there are models with temperature compensation, but they are more expensive). And also the conductivity depends on the active reaction of water (pH). With prolonged use, sulfation of electrodes is possible, which also distorts the readings of the device (there are models that use alternating current for measurement, but they are also more expensive). If you follow the manufacturer’s recommendations, the need for frequent quick measurements and the ability to periodically monitor the readings of the device more accurate methods, such a device is simply not replaceable.

Water hardness test strips
Specially designed for aquarists and usually of foreign manufacture. Very convenient and simple. There are also kits in which the reagent is added drop by drop to a certain amount of water until the color of the water changes. Calculation of hardness is carried out according to the attached instructions. The disadvantage is that they are rarely on sale, at least near me. If your nearest pet store has them – you will not regret buying these kits.
The most accessible, cheapest and most accurate at home, without the use of sophisticated chemical or electronic equipment, is the method of
How to find out the hardness of water with ordinary laundry soap

This method was described in his book by I. Sheremetev. This method is based on the fact that laundry soap, as well as any other soap is difficult to soap in hard water. And only when soap binds excess calcium and magnesium salts – soap foam appears.
To determine the hardness of water, weigh one gram of laundry soap, crush it and carefully dissolve it in a small amount of hot distilled water so that no foam is formed. Distilled water can be bought in auto stores. It is used to add it to the battery when the electrolyte concentration increases.
Analyzing
Then pour the soap solution into a cylindrical beaker and add distilled water to the level of 6 centimeters if the soap is 60% or to the level of 7 centimeters if the soap is 72%. The percentage of soap content is indicated on the bar. Now each centimeter of soap solution level contains the amount of soap able to bind hardness salts, the amount of which corresponds to 1°dH in 1 liter of water. Next, in a liter jar pour half a liter of water under study. And stirring continuously, little by little add our soap solution from the glass into the jar with the water under test.
Evaluation of results
At first, only gray flakes will appear on the surface. Then colorful soap bubbles will appear. The appearance of a stable white soap foam indicates that all hardness salts in the tested water are bound. Now we look at our beaker and determine how many centimeters of solution we had to pour out of the beaker into the water under study. Each centimeter bound in half a liter of water the amount of salts corresponding to 2°dH. Thus, if you had to pour 4 centimeters of soap solution into the water before the appearance of foam, the hardness of the tested water is 8°dH.
If you have poured all the soap solution into the water and still no foam appears, it means that the hardness of the test water is greater than 12°dH. In this case, dilute the tested water twice with distilled water. And analyze again. Now the obtained result of hardness should be multiplied by two. The obtained value will correspond to the hardness of the tested water.
Uncertainty accounting
A standard beaker has a volume of 200 – 250 ml. The height is about 10 centimeters. The lower diameter is from 55 millimeters, the upper diameter is 73 millimeters. That is, the average diameter is about 63 millimeters. If you can find a chemical cylindrical beaker with suitable characteristics (diameter of about 6 centimeters), your measurements will be more linear. If a cylinder is not available, you can also use a cone beaker. In this case, an increase in the measurement error is inevitable. But after all, the method itself is not a benchmark of accuracy.
Achievable accuracy
With accuracy to thousandths of a degree hardness can not be determined by this method, but to assess the sharp departure of the total hardness from the norm with an accuracy of 1 -2 °dH is quite possible. In this case, it is the difference between the upper and lower diameters of the vessel in which the soap solution is located and gives this error of 1-2 degrees. In addition to the variable diameter, the quality of the soap, distilled water, and your experience with this technique affects the error. If for pH error in 1 – 2 degrees can be catastrophic, then for the total hardness of such a spread of readings is quite acceptable, it will not lead to the death or health of the inhabitants of your aquarium.
With certain experience, the error of the method is about 1 – 2 °dH. That is quite acceptable for our purposes. Given the simplicity and availability of the method, it certainly deserves attention.
There are other methods of determining water hardness. But some of them are technically complicated, others require sophisticated equipment, and others are not safe for home use. Therefore, I do not mention them here. How to change water hardness is discussed in the article “How to remove water hardness at home”.