Water pH or the hydrogen value is one of the most widely used characteristics of aqueous solutions in both engineering and biology. The importance of the hydrogen value can be imagined if we remember that a decrease in pH in the internal environment of the body by just half a unit will lead to death.
The importance of water pH for a living organism
Living organisms have adapted to certain conditions of existence. And in these conditions to which they are adapted, they can maintain homeostasis indefinitely. In conditions sharply different from those to which the organism is adapted, its ability to maintain homeostasis falls sharply. The aquatic environment has certain characteristics, one of which is the hydrogen index. The closer the environmental conditions are to the ideal conditions for a given species, the easier it is for the organism to maintain a constant internal environment. When the organism feels good, it gets sick less and lives longer.
It is often preferable for living organisms that the hydrogen value of the environment is close to a neutral value. All this leads to the need to control the acidity of the solutions surrounding us and aquarium water in particular. Let’s try to find the most acceptable way for home conditions.
What is pH?
The hydrogen exponent is the negative logarithm of the hydrogen ion concentration. pH = – ln[H+] . The concept of hydrogen index was introduced by Danish chemist Sørensen in 1909. It is denoted by the first letters of the English words potentia hydrogeni (hydrogen strength) or pondus hydrogenii (hydrogen weight). Pronounced “pe ash” in Russian transcription, “pi h” in English.
In pure water at 25 degrees Celsius, the concentration of hydrogen ions [H+] and hydroxide ions [OH-] are the same and equal to 10-7 mol/L.
Which follows from the equation [H+] *[OH-] =10-14 mol²/l² at 25 degrees Celsius. If the concentrations of both kinds of ions in water are equal [H+] =[OH-], then the solution has a neutral reaction. That is, pH=7. If the concentration of hydrogen ions[H+] increases, the pH value decreases and becomes less than seven, and the acidity of the solution increases and the alkalinity decreases accordingly.
If the concentration of hydroxide ions [OH-] increases, the pH value increases (becomes greater than 7). The alkalinity of the solution increases and the acidity decreases.
Modern pH scale
The pH scale used today is divided into 14 divisions.
0- minimum (very acidic environment). 7- neutral. 14 – maximum (highly alkaline environment). To be fair, it should be said that pH values can go beyond the currently accepted scale and can be less than zero and more than 14. But this is quite rare in engineering, and even more so in biology. So this representation of one of the environmental parameters suits everyone.
В биологии и технике на сегодняшний день существует несколько основных способов для определения рН. Рассмотрим каждый из них подробнее.
Basic methods for determining the pH of water
Determination of pH of water using color organic indicators.
Many organic substances can change their color depending on their acidity. The most famous such “indicator” is tea. When you drop a slice of lemon into a glass of strong brown tea, the tea in the glass becomes much lighter in color. When the pH decreases, that is, when the acidity increases due to citric acid, the tea changes its color to a lighter color.
Another equally famous “indicator” is the juice of table beets. Housewives have long known its ability to take a more intense red color in an acidic environment. After the borscht is almost ready and has an orange color add a little vinegar to it and right before your eyes your dish will turn bright red.
Of course, such indicators are not currently used in engineering and biology for various reasons.
Nowadays, the best known indicators in chemistry are litmus, phenolphthalein, methyl orange (methyl orange) and a number of others.
These indicators can have two colors depending on the acidity of the medium. The color for each indicator can vary between 1 and 2 pH units.
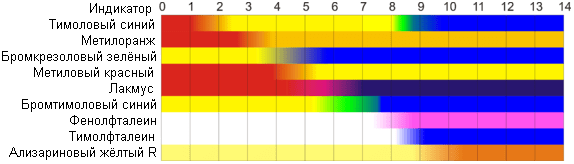
Consequently, this method of determining the hydrogen index has a number of disadvantages. First, acidity can be determined only in a certain small range of pH values. This disadvantage is sometimes circumvented by preparing a universal indicator – a mixture of several of the above indicators. But this indicator still does not cover the entire scale of hydrogen activity.
Secondly, the use of indicator color change for the determination of hydrogen ion activity is very difficult and sometimes impossible in turbid and colored media
Determination of water pH Acid basic titration

Using this method, the hydrogen ion concentration can be accurately determined. The method in general outline is as follows. In a certain volume of the solution under study with an indicator drop by drop add a solution with a known concentration of hydrogen ions until the color of the solution changes. That indicates that the amount of titrant necessary for the complete completion of the reaction has been added to the solution under study. Further, knowing the exact concentration of titrant and its volume added before the color change, it is possible to calculate the concentration of hydrogen ions in the test solution.
Despite the accuracy of the method, it is still not quite suitable for home use. First of all, it requires additional chemical equipment. And we don’t want to turn our apartment into a chemical laboratory.
Secondly, it requires certain skills in the preparation of chemical solutions of exact concentration, as well as the disadvantages include the need for chemical calculations. That is, the method is cumbersome and not operational.
And thirdly, analysis is also difficult in colored and turbid liquids.
However, if you have access to a chemistry lab, this is probably the only method for initial calibration of instruments and test strips and further verification of their readings.
pH metrology using an electronic instrument.

By far, probably the most convenient way to determine the hydrogen index is by electronic pH metrology.
It is based on the measurement of the EMF of a galvanic circuit, which includes a special glass electrode. The potential of this electrode depends on the concentration of hydrogen ions in the solution under study.
pH meters have a long history. The development of such measuring equipment was especially stimulated in 1940 – 1950 by the defense order. Due to the urgent need for such devices to control technological processes at harmful production facilities and in aggressive environments.
As a result of research, there have been a number of discoveries, many successes and just as many mistakes. As a result, modern compact devices with electrode pair, in which the glass electrode is the main electrode, have appeared. The chlorosilver electrode plays an auxiliary role.
The electronic circuit of the device has also gone a long way from the bridge circuit to modern circuits built on analog-to-digital converters and controlled by microprocessors.
Electronic water pH meter suitable for everything. Covers the entire range of hydrogen ion concentrations. It has a measurement accuracy of approx. 0.01. You can measure the hydrogen index in turbid and colored media. The only disadvantage is the price.
Although now on sale there are pH meters designed specifically for amateur aquarists and gardeners. These devices have sufficient accuracy at a relatively low price.
Some conclusions
To conclude this short review article on a large and broad topic, we would like to conclude that today either pH test strips can be recommended for determining pH at home. Or pH meters – electronic devices with glass electrodes and processor control.
Test strips and pH meters require separate review articles. In addition, it is possible to make your own pH test strips. And there are designs of homemade pH meters.